Time Left - 12:00 mins
BARC 2019: Thermodynamics Nuclear Quiz-2
Attempt now to get your rank among 729 students!
Question 1
The steam is expanded isentropically in steam turbine from 20 bar, 350°C to 0.08 bar and then it condensed to saturated liquid water in condenser. The pump feeds back the water into boiler. Neglect losses in the processes, the cycle efficiency (in percentage) is
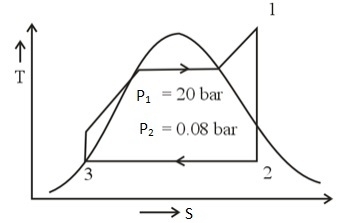
Given data: At P = 0.08 bar, hfg = 2403.1 kJ/kg, sfg = 7.6361 kJ/kg K, vf = 0.001008 m3/kg
h1 =3159.3 kJ/kg, s1 = 6.9917 kJ/kg K, h3 = 173.88 kJ/kg, s3 = 0.5926 kJ/kgK,

Given data: At P = 0.08 bar, hfg = 2403.1 kJ/kg, sfg = 7.6361 kJ/kg K, vf = 0.001008 m3/kg
h1 =3159.3 kJ/kg, s1 = 6.9917 kJ/kg K, h3 = 173.88 kJ/kg, s3 = 0.5926 kJ/kgK,
Question 2
An ideal refrigerator which works between the temperature limits of 600o C and 1000o C, is having COP 5.If it is used to operate as a heat pump between the same temperature limits, then the COP will be
Question 3
In a cooling tower, the DBT and WBT of air is 28°C and 23°C respectively while the temperature of water at inlet and outlet states is 32°C and 24°C respectively. The approach and range (in °C) in cooling tower respectively is
Question 4
In a single heater regenerative cycle (as shown in fig.), the 1 kg of steam enters the turbine at 25 bar, 400 and the exhaust pressure is 0.1 bar. The feed water heater of direct contact type operates at 5 bar.
and the exhaust pressure is 0.1 bar. The feed water heater of direct contact type operates at 5 bar.
The values of enthalpies at different states are shown in table:

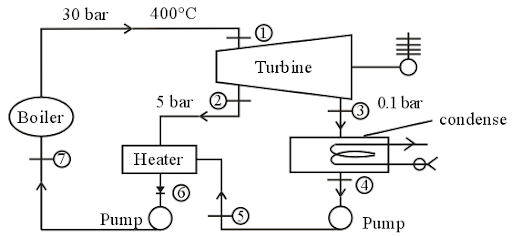
If the entropy at states 1, 4 and 6 are 6.9212 KJ/kgK, O.6493 KJ/kgK and 1.867 KJ/kgK respectively, then the increase in mean temperature of heat addition (in ) after heat regeneration is
) after heat regeneration is
The values of enthalpies at different states are shown in table:

If the entropy at states 1, 4 and 6 are 6.9212 KJ/kgK, O.6493 KJ/kgK and 1.867 KJ/kgK respectively, then the increase in mean temperature of heat addition (in
Question 5
What is the mean effective pressure of air standard cycle which is 53% efficienct and having 1200kJ/kg of heat supplied, with compression ratio and swept volume of 7 and 0.656  respectively ?
respectively ?
Question 6
An air standard diesel cycle has compression ratio of 15. The pressure and temperature at the beginning of compression stroke is 1bar and 25 °C.The maximum temperature is 2500 °C. Determine the thermal efficiency (%)of the engine.
Question 7
Which of the following statements is correct?
1) All the reversible cycles operating under same maximum and minimum temperature and working substance have same efficiency.
2) Entropy of an internally irreversible and adiabatic process is always positive.
3) An internally reversible adiabatic process is called isentropic process.
4) For an internally irreversible process change in entropy can be positive, negative or zero.
1) All the reversible cycles operating under same maximum and minimum temperature and working substance have same efficiency.
2) Entropy of an internally irreversible and adiabatic process is always positive.
3) An internally reversible adiabatic process is called isentropic process.
4) For an internally irreversible process change in entropy can be positive, negative or zero.
Question 8
Nitrogen gas of 0.05m3 volume is contained in a piston cylinder device having initial temperature and pressure of 1bar and 25 ºC. The gas is compressed isothermally and reversibly until the pressure is 5bar. Calculate change in entropy for the process.
Question 9
A copper rod is of length 1 m and diameter 0.02 m. One end of the rod is at 150 °C, and the other at 0 °C. The rod is perfectly insulated along its length and the thermal conductivity of copper is 380 W/m-K. Calculate the rate of entropy production due to irreversibility of this heat transfer.
Question 10
2 kg of air initially at 7 bar pressure and 400 K expands polytropically (PV1.2 = Constant) until the pressure is reduced to one-fifth value.The change in entropy (in kJ/K ) is _____. (Take R= 0.287 kJ/kgK and γ=1.4)
- 729 attempts
- 8 upvotes
- 7 comments
May 18ESE & GATE ME

