Time Left - 15:00 mins
AIIMS 2019 Physics Heat and Thermodynamics Revision Quiz 2
Attempt now to get your rank among 202 students!
Question 1
Which one of the following is a reversible process:
Question 2
Statements:
(i)According to Charles Law, V/T= constant while pressure remains constant
(ii)According to Boyle’s Law, P/V= constant while Temperature remains constant
(iii)According to Dalton’s Law, P=P1+P2+…. When Temperature and Volume remain Constant
(iv) According to Ideal Gas Equation, Pn=VRT (where P is Pressure, n is Volume, V is no. of moles, T is Temperature, and R is universal gas constant)
Mark the set containing all correct statements or all incorrect statements:
Question 3
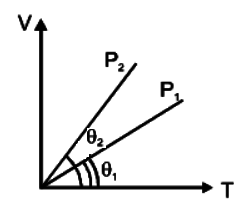
In the given (V–T) diagram, what is the relation between pressure P1 and P2 ?
Question 4
For a gas  =0.67. This gas is made up of molecules which are
=0.67. This gas is made up of molecules which are
Question 5
An ideal gas heat engine operates in a Carnot cycle between 227oC and 127oC. It absorbs 6 kcal at the higher temperature. The amount of heat (in kcal) converted into work is equal to:
Question 6
Consider two rods of same length and different specific heats(s1, s2),thermal conductivities (K1, K2) and areas of cross section (A1, A2)and both having temperatures(T1, T2) at their ends. If their rate of loss of heat due to conduction is equal, then:
Question 7
For a black body at temperature 727 °C, its radiating power is 60 W and temperature of surrounding is 227 °C. If the temperature of the black body is changed to 1227 °C, then its radiating power will be:
Question 8
The temperature of a metal object drops from 75oC to 65oC in 2 minutes in a room with ambient temperature of 30oC. What will be the time taken to cool down the same object from 50oC to 40oC in the same room?
- 202 attempts
- 0 upvotes
- 7 comments
Tags :
Aug 9NEET & AIIMS

