Time Left - 20:00 mins
NEET 2020 Chemistry : Chemical Kinetics and Nuclear Chemistry Quiz -2(21.06.2019)
Attempt now to get your rank among 293 students!
Question 1
Half-life period of a first order reaction is 1386 s. The specific rate constant of the reaction is
Question 2
Mechanism of a hypothetical reaction
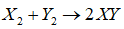 is given below:
is given below:
(i)
(ii)
(iii)
The overall order of the reaction will be:
(i)
(ii)
(iii)
The overall order of the reaction will be:
Question 3
The rate constants k1 and k2 for two different reactions are 1016. e-2000/T and 1015. e-1000/T, respectively. The temperature at which k1= k2 iS
Question 4
The reaction of hydrogen and iodine monochloride is given as :
H2(g) + 2ICl(g) → 2HCl(g) + I2(g)
This reaction is of first order with respect to H2(g) and ICl(g), following mechanisms were proposed :
Mechanism A :
H2(g) + 2ICl(g) → 2HCl(g) + I2(g)
Mechanism B :
H2(g) + ICl(g) →HCl(g) + HI(g); slow
HI(g) + ICl(g) →HCl(g) + I2(g); fast
Which of the above mechanism(s) is more appropriate with the given information about the reaction?
H2(g) + 2ICl(g) → 2HCl(g) + I2(g)
This reaction is of first order with respect to H2(g) and ICl(g), following mechanisms were proposed :
Mechanism A :
H2(g) + 2ICl(g) → 2HCl(g) + I2(g)
Mechanism B :
H2(g) + ICl(g) →HCl(g) + HI(g); slow
HI(g) + ICl(g) →HCl(g) + I2(g); fast
Which of the above mechanism(s) is more appropriate with the given information about the reaction?
Question 5
The rate of a first-order reaction is 0.04 mol l‑1 s-1 at 10 seconds and 0.03 mol l-1 s-1 at 20 seconds after initiation of the reaction. The half-life period of the reaction is:
Question 6
For the reaction, N2 + 3H2→2NH3, if =2 x 10-4 mol L-1s-1, the value of
=2 x 10-4 mol L-1s-1, the value of  would be
would be
Question 7
Consider the reaction
N2(g) + 3H2(g) → 2NH3(g)
The equality relationship between and
and  is :
is :
N2(g) + 3H2(g) → 2NH3(g)
The equality relationship between
Question 8
The bromination of acetone plat occurs in acid solution is represented by this equation.
CH3COCH3(aq)+ Br2(aq) → CH3COCH2Br(aq) + H+(aq) + Br-(aq)
These kinetic data were obtained for given reaction concentrations.
Based on these data, the rate equation is
CH3COCH3(aq)+ Br2(aq) → CH3COCH2Br(aq) + H+(aq) + Br-(aq)
These kinetic data were obtained for given reaction concentrations.
Initial concentrations, M  |
Initial rate, Ms-1  |
Based on these data, the rate equation is
Question 9
In a zero order reaction for every 10 ° rise of temperature, the rate is doubled. If the temperature is increased from 10 °C to 100 °C, the rate of the reaction will become
Question 10
If 50% of a reaction occurs in 100 second and 75% of the reaction occurs in 200 second, the order of this reaction is :
- 293 attempts
- 3 upvotes
- 10 comments
Tags :
Mar 26NEET & AIIMS

