Time Left - 20:00 mins
JEE 2020 Physics:Thermodynamics Quiz (09.07.2019)
Attempt now to get your rank among 125 students!
Question 1
In thermodynamic process pressure of a fixed mass of gas is changed in such a manner that the gas releases 30 joule of heat and 18 joule of work was done on the gas. If the initial internal energy of the gas was 60 joule, then, the final internal energy will be :
Question 2
A cylinder made of perfectly non conducting material closed at both ends is divided into two equal parts by a heat proof piston. Both parts of the cylinder contain the same masses of a gas at a temperature t0 = 27° and pressure P0 = 1 atm. Now if the gas in one of the parts is slowly heated to t = 57°C while the temperature of first part is maintained at t0 the distance moved by the piston from the middle of the cylinder will be (length of the cylinder = 84 cm)
Question 3
Two identical vessels A & B contain equal amount of ideal monoatomic gas. The piston of A is fixed but that of B is free. Same amount of heat is absorbed by A & B . If B’s internal energy increases by 100 J the change in internal energy of A is

Question 4
When a system is taken from state ’a’ to state ‘b’’ along the path ‘acb’’, it is found that a quantity of heat Q = 200 J is absorbed by the system and a work W = 80 J is done by it. Along the path ’adb’, Q = 144J. If Ua = 40 J, value of Ub will be
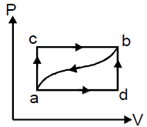

Question 5
In a thermodynamic process, 185 moles of an ideal diatomic gas is kept in a cylinder at temperature . On supplying heat to the gas, its temperature remains constant but 20 moles get dissociated into atoms. Find the heat supplied to the gas. Assume that
. On supplying heat to the gas, its temperature remains constant but 20 moles get dissociated into atoms. Find the heat supplied to the gas. Assume that
Question 6
Henry has a solid steel block. The solid contains a hole where the bronze pin is to be fit. A bronze pin having temperature ‘T’ is inserted into the hole. The size of the hole is less than that of the diameter of the pin. The required changes for an exact fit of the pin into the hole is
Question 7
Three processes from a thermodynamic cycle as shown on P-V diagram for an ideal gas. Process 1 → 2 takes place at constant temperature (300 K). Process 2 → 3 takes place at constant volume. During this process 40J of heat leaves the system. Process 3 → 1 is adiabatic and temperature T3 is 275K. Work done by the gas during the process 3 → 1 is

Question 8
When unit mass of water boils to become steam at 100°C, it absorbs Q amount of heat. The densities of water and steam at 100°C are ρ1 and ρ2 respectively and the atmospheric pressure is p0. The increase in internal energy of the water is
Question 9
A ideal monoatomic gas is carried around the cycle ABCDA as shown in the fig. The efficiency of the gas cycle is

Question 10
One mole of an ideal gas at temperature T1 expands slowly according to the law P/V = constant. If the final temperature is T2, work done by the gas equals
- 125 attempts
- 4 upvotes
- 1 comment
Tags :
Jul 9JEE & BITSAT

