Time Left - 10:00 mins
NEET 2020 Physics: Kinetic Theory of Gaseous -01 Quiz
Attempt now to get your rank among 638 students!
Question 1
The molar specific heats of an ideal gas at constant pressure and volume are denoted by Cp and Cv, respectively. If γ = 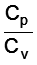 and R is the universal gas constant, then Cv is equal to :
and R is the universal gas constant, then Cv is equal to :
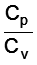 and R is the universal gas constant, then Cv is equal to :
and R is the universal gas constant, then Cv is equal to : Question 2
For a gas  =0.67. This gas is made up of molecules which are
=0.67. This gas is made up of molecules which are
Question 3
A balloon contains 500m3 of helium at 27 °C and 1 atmosphere pressure. The volume of the helium at - 30 C temperatures and 0.5 atmosphere pressures will be
Question 4
A given sample of an ideal gas occupies a volume V at a pressure p and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas?
Question 5
The molecules of a given mass of a gas have R.M.S. velocity of 200 ms-1 at 27 °C and 1.0x105 Nm-2 pressure. When the temperature and the pressure of the gas are respectively, 127 °C and 0.5 x 105 Nm-2, the RMS velocity of its molecules in ms-1 is
- 638 attempts
- 5 upvotes
- 40 comments
Tags :
Mar 3NEET & AIIMS

