The word Thermodynamics originates from the Greek words therme (heat) and dynamic (power), so thermodynamics can be referred as the science in which study of the transfer of heat takes place.
“Thermodynamics is the study of energy transfer and its transformation and effects on the physical properties of substance”.
System
A system can be defined as a quantity of matter (control mass) or a region (control volume) in space selected for the study.
SURROUNDING- Everything external to the system is known as surrounding or the environment. Energy interaction is studied between the system and surroundings i.e. every energy leaving the system will be absorbed by the surrounding and vice versa.
BOUNDARY- An imaginary or real surface that demarcates the system from its surroundings is known as the boundary. The boundary is the surface of contact between the system and surrounding, thus, shared by both the system and the surroundings. Mathematically, the boundary has no thickness, and can neither occupy any volume in space nor contain any mass. The boundary may either be moving or fixed.
Universe- A system and its surroundings together constitute the universe. Everything is contained in the universe, so everything occurring whether energy transfer or transformation or losses remains inside the universe.
Types of System
Depending upon the mass and energy interaction.
(i) Open System – When there is mass as well as energy transfer across the boundary, that type of system is called an Open system. Example - air compressor, boiler, pump, IC engine with valve open, etc. The majority of engineering devices come under this category.

(ii) closed system –When in a system, the mass remains fixed or constant but there may be energy transfer into or out of the system i.e. no mass transfer occurs across the system boundary but only energy transfer. Example – Tea in kettle, automobile engine with valve closed etc.

(iii) Isolated system - When there is no mass and energy interaction taking place between the system and the surroundings, such systems are called isolated systems. It is of fixed mass and energy, and there is no mass or energy interaction across the system boundary. Example – thermo-flask, Universe

Macroscopic v/s Microscopic Approach
Macroscopic Approach - When a certain quantity of matter is considered, without getting into molecular level, such systems are called Isolated systems (also called as classical approach). Every property will be the average of that property of each molecule passing through that space.
Microscopic Approach - When study is made on a molecular level as matter is composed of a large number of molecules, such approach is called a Microscopic approach. The behavior of the gas is determined by considering the behavior of each molecule.
CONCEPT OF CONTINUUM- The concept of the continuum is the idealization of the continuous description of matter where the properties of the matter are considered as continuous functions of space. The space between the molecules (mean free path) is almost zero or negligible when compared to the size of the system.
Extensive Properties v/s Intensive Properties
(i) Extensive Properties – The properties dependent on mass are known as extensive properties (sometimes known as extrinsic properties). Since, the mass of the specimen changes, the value of extensive properties will also change according to it.
Example – Volume, Enthalpy, Weight, etc.
(ii) Intensive Properties – Extrinsic properties per unit mass, are intensive properties. These properties are independent of the mass of the system (also known as intrinsic properties). It is a system property, independent of quantity.
Example – Pressure, Density, Viscosity, specific energy, specific enthalpy etc.
Thermodynamic Equilibrium
When no change in macroscopic properties is observed, a system is said to be in a state of thermodynamic equilibrium. A system will be in a state of thermodynamic equilibrium if the following conditions are met:
(i) Mechanical Equilibrium - without the presence of an unbalanced force within the system itself and also between the system and the surroundings.
(ii) Chemical equilibrium - an absence of any chemical reaction or transfer of matter from one part of the system to another.
(iii) Thermal equilibrium - When a system exists in mechanical as well as a chemical equilibrium when separated from its surroundings by a diathermic wall (diathermic means ‘which allows heat to flow’).
Even when one of these conditions is not met, the system can't be in thermodynamic equilibrium.
The thermodynamic properties are defined only for thermodynamic equilibrium states.
PROCESS - Any change of state that a system undergoes, from one equilibrium state to another equilibrium state is known as a process.

PATH - The succession of states passed through during a change of state from an initial condition to the final required condition, is called the path of the change of state.

CYCLE - A series of changes in states of a system, such that the final point of the system coincides with the initial point is termed as a cycle.

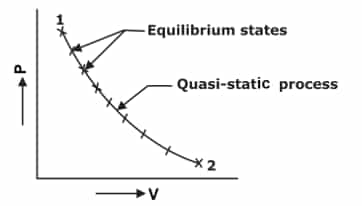
QUASI-STATIC PROCESS- The meaning of ‘Quasi’ is ‘almost’ and the meaning of ‘Static’ is ‘at rest’. The characteristic feature of a quasi-static process is its infinite slowness. A process that is the locus of all the equilibrium states the system passes through from an initial condition to the final desired condition is known as a quasi-static process. Every state of the system through which it passes during this process is an equilibrium state.
Reversible and Irreversible Process
A reversible process is a process that can be reversed without causing any permanent change in the surroundings. That is, both the system and the surroundings are returned to their initial states if the given process is reversed. In the property diagrams, reversible processes are shown by continuous line or curve whereas irreversible processes are shown by dotted line or curve.
Reversible processes are actually only theoretical. They are a mere idealization of actual processes. Processes that are not reversible are termed irreversible processes.
PURE SUBSTANCE- A substance that has a uniform and invariable chemical composition throughout its mass is known as a pure substance.
Examples: Atmospheric air, steam-water mixture, ammonia, etc.
IDEAL GAS EQUATION - Ideal (perfect) gas equation is a unique equation of state, which is applicable specifically to ideal gases. The molecular forces of attraction between gas molecules are negligible in an ideal gas. The volume of the molecules should be negligible compared to the total volume for a perfect gas. Following is the perfect or ideal gas equation :
PV=nRT
Where:
P = Absolute Pressure = atmospheric pressure + Gauge pressure (in pascal)
V = Volume in m3
R= Universal Gas constant = 8.314KJ/Kmol-K
T = Absolute temperature in kelvin
n = number of moles (in k-mol)
Boyle’s Law- When the temperature is kept constant, the variation of pressure is such that for a volume of a given mass of gas it varies inversely.
Charles Law- When the pressure remains constant, then the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature. It is the basis of temperature measurement.
ZEROTH LAW OF THERMODYNAMICS
- It is the basic law of thermodynamics which defines the temperature.
- It defines that “If thermal equilibrium of body P exists with a body Q and body R separately, then there must be thermal equilibrium between Q and R also”.

Energy
(i). In a thermodynamic system, Energy interaction can be in three ways namely work, heat, and by mass.

(ii). There is only work transfer and Heat transfer interaction between a closed system and surroundings without any mass transfer, while work, Heat, and mass transfer interaction all occur in the open system (because mass also carries energy).
You can avail of BYJU’S Exam Prep Online classroom program for all AE & JE Exams:
BYJU’S Exam Prep Online Classroom Program for AE & JE Exams (12+ Structured LIVE Courses)
You can avail of BYJU’S Exam Prep Test series specially designed for all AE & JE Exams:
BYJU’S Exam Prep Test Series AE & JE Get Unlimited Access to all (160+ Mock Tests)
Thanks
Team BYJU’S Exam Prep
Download BYJU’S Exam Prep APP, for the best Exam Preparation, Free Mock tests, Live Classes.




Comments
write a comment